
Uranium 236 is formed in the nuclear fuel from uranium 235, after neutrons captures that did not cause fission. Submitted to the flow of neutrons from a reactor, it undergoes fission, but rarely turns into uranium 232 by a specific capture reaction (n, 2n) that triggers the expulsion of two neutrons. Uranium-233 is formed by a similar capture by a nucleus of natural thorium, followed by two radioactive decays. Uranium-236 is formed by a simple radiative capture by a nucleus of uranium-235 non followed by fission. The isotopes 236, 233 and 232 of uranium are formed in reactors from captures of neutrons not followed by fission. In a natural sample of uranium, these nuclei are present in the unalterable proportions of the radioactive equilibrium of the uranium-238 filiation at a ratio of one atom of uranium-234 for 18 800 atoms of uranium- 238, so that the two isotopes contribute equally to the radiations emitted by uranium. Uranium 234 is the first long-lived descendant of uranium-238. If humans had been present at the beginning of Earth, they would not have needed to enrich uranium to make atomic bombs or operate their reactors! The 0.7% observed today are a pale residue of this past abundance. At the time of the formation of Earth, U-235 was 85 times more abundant. Its very long half-life (or period), 700 million years, is however, 6.5 times shorter than that of the isotope 238. This very rare isotope, present at the concentration of 0.7% in natural uranium, is thus a highly strategic and coveted material. Uranium 235, the only existing fissile nucleus found in natural uranium, is used as a nuclear fuel in reactors and as an explosive for nuclear weapons. The purpose of the fourth-generation breeder reactors is to recover this fantastic potential. This impressive potential of fission energy remains still largely unexploited. Hardly fissile, U-238 contributes to the operation of reactors and production of electricity through this plutonium. Neutron capture by this nucleus leads to the formation of fissile plutonium-239 in a reactor. Its very long period says it is still present in the Earth crust. Uranium 238, which alone constitutes 99.3% of natural uranium has the longest lifetime: its period is 4.5 billion years, about the age of Earth. © ANL/Uranium fact sheet © Three naturally occurring isotopes

Specific activities (activities given for 1 gram) are inversely proportional to half-lives. The left columns of the table show however the presence of a low energy gamma radiation and rare decays beta.
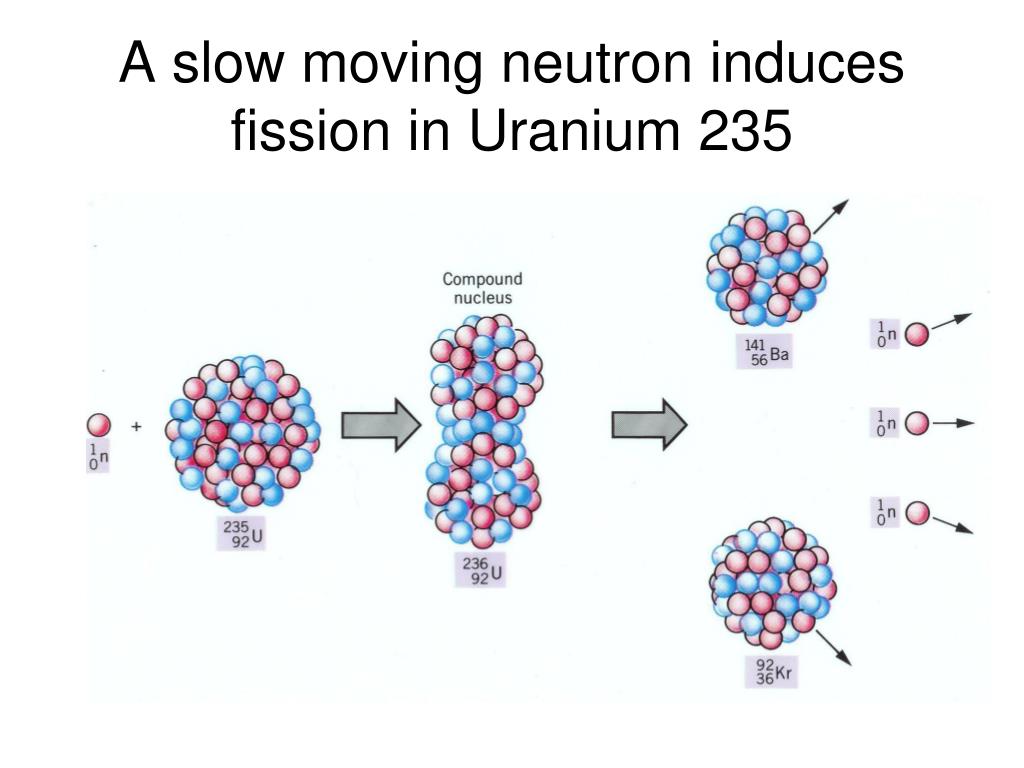
All are alpha emitters of 4 to 5 MeV of energy. The main isotopes of uranium contained in this table have extremely long lifetimes with the exception of uranium 232. Comparison of radioactive properties of uranium isotopes


 0 kommentar(er)
0 kommentar(er)
